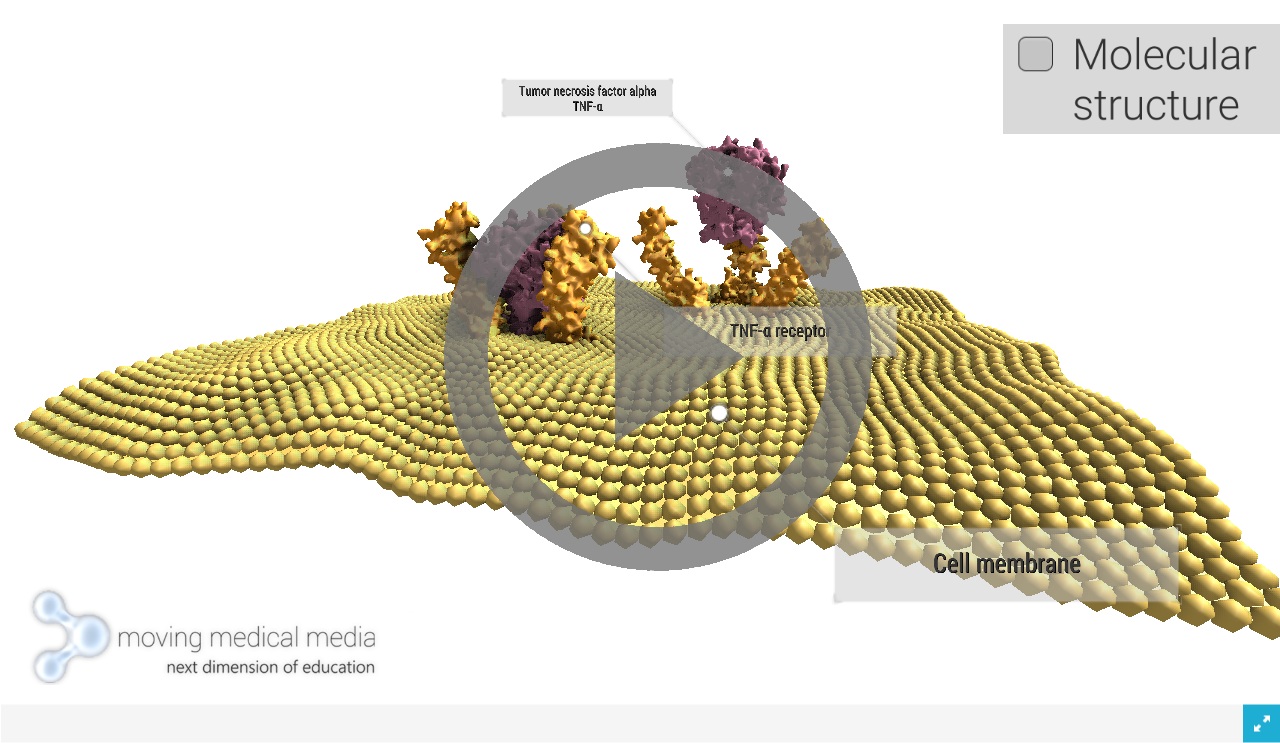
The principle of an antitumoral response of the immune system in vivo has been recognized already about 100 years ago by the physician William B Coley. A soluble cytokine named tumor necrosis factor (TNF) has been identified 30 years later. This cytokine is produced upon activation by the immune system, able to exert significant cytotoxicity on many tumor cell lines and to cause tumor necrosis in certain animal model systems. In 1984, the cDNA of TNF was cloned and structural and functional homology to lymphotoxin (LT) α was realized. Several years later, two membrane receptors, each capable of binding both cytokines, were identified. Subsequently, it was recognized that TNF is the prototypic member of a large cytokine family, the TNF ligand family (Wajant, 2003). The tumor necrosis factor superfamily, composed of 19 ligands and 29 receptors, plays very different roles in the body. All members of the TNF superfamily, without exception, exhibit pro-inflammatory activity, in part through activation of the transcription factor NF-κB. Several members of the TNF superfamily exhibit proliferative activity on hematopoietic cells, in part through activation of various mitogen-activated kinases, and some members of this family play a role in apoptosis. Some members of the TNF superfamily have also been reported to play a role in morphogenetic changes and differentiation. Most members of the TNF superfamily have both beneficial and potentially harmful effects. Although TNF-α, for example, has been linked with physiologic proliferation and differentiation of B cells under steady-state conditions, it also has been linked with a wide variety of diseases, including cancer, cardiovascular, neurologic, pulmonary, autoimmune, and metabolic disorders (Aggarwal,2012). TNFα is mainly produced by macrophages, whereas TNFβ is mainly produced by T lymphocytes. Also other cells can also express TNFα and TNFβ at low levels. TNF exerts many important physiological and pathological actions. TNF causes tumor cell necrosis and apoptosis. Additionally, TNF is a key mediator of both acute and chronic systematic inflammatory reactions. TNF not only induces its own secretion, but it also stimulates the production of other inflammatory cytokines and chemokines. TNF plays a central role in autoimmune diseases such as rheumatoid arthritis (RA), inflammatory bowel diseases including Crohn’s disease and ulcerative colitis, multiple sclerosis, systemic lupus erythematosus and systemic sclerosis. Finally, TNF has emerged as an important risk factor for tumorigenesis, tumor progression, invasion and metastasis. TNF is a key intermediary of cancer-associated chronic inflammation (CHU, 2013). [1]
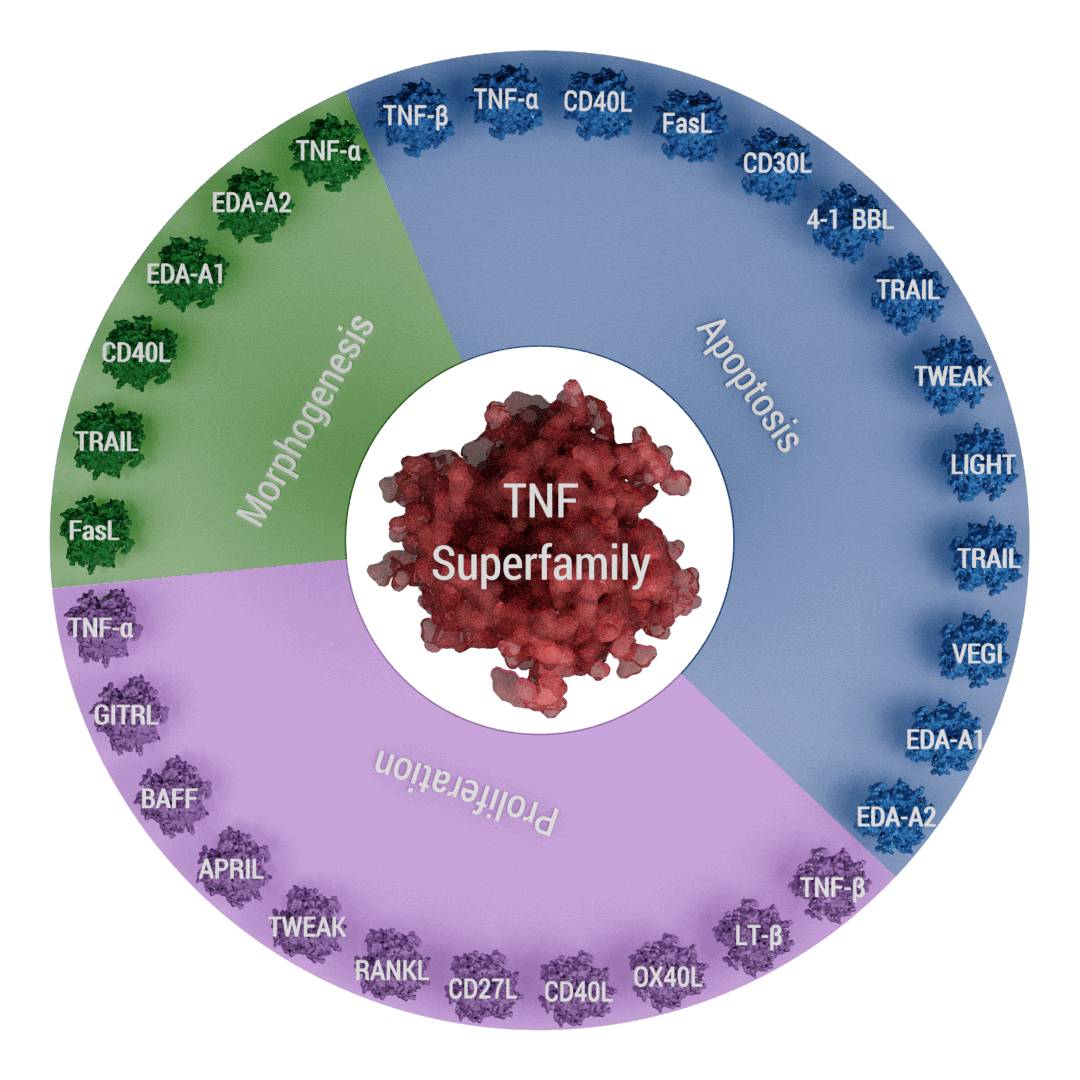 Roles of various members of the TNF superfamily in inflammation, cellular proliferation, apoptosis, and morphogenesis. All members of the TNF superfamily exhibit pro-inflammatory activity, in part through activation of the transcription factor NF-κB (full red circle); OX40L, CD40L, CD27L, APRIL, and BAFF exhibit proliferative activity in part through activation of various mitogen-activated kinases (sky blue); TNF-α, TNF-β, FasL, and TRAIL control apoptosis
(bluish-green); and EDA-A1, EDA-A2, TNF-α, FasL, and TRAIL regulate morphogenesis (green) (adapted from Aggarwal, 2012).
Roles of various members of the TNF superfamily in inflammation, cellular proliferation, apoptosis, and morphogenesis. All members of the TNF superfamily exhibit pro-inflammatory activity, in part through activation of the transcription factor NF-κB (full red circle); OX40L, CD40L, CD27L, APRIL, and BAFF exhibit proliferative activity in part through activation of various mitogen-activated kinases (sky blue); TNF-α, TNF-β, FasL, and TRAIL control apoptosis
(bluish-green); and EDA-A1, EDA-A2, TNF-α, FasL, and TRAIL regulate morphogenesis (green) (adapted from Aggarwal, 2012).
TNF alpha receptor
Tumor necrosis factor blockers revolutionized the care of patients with multiple immune disorders, including rheumatoid arthritis, psoriasis, psoriatic arthritis, ankylosing spondylitis, and inflammatory bowel disease - Crohn's disease and ulcerative colitis. Modulation of the immune response with TNF blockers is not a new treatment strategy for many inflammatory disorders. Relatively little is known about their mechanisms of action in patients. Understanding the mode of action, pharmacology, and pharmacokinetics of infliximab and adalimumab (both monoclonal anti-TNF antibodies), and etanercept (soluble TNF receptor-Fc fusion protein), may therefore enable us to account for their differing clinical profiles (Gottlieb,2007).
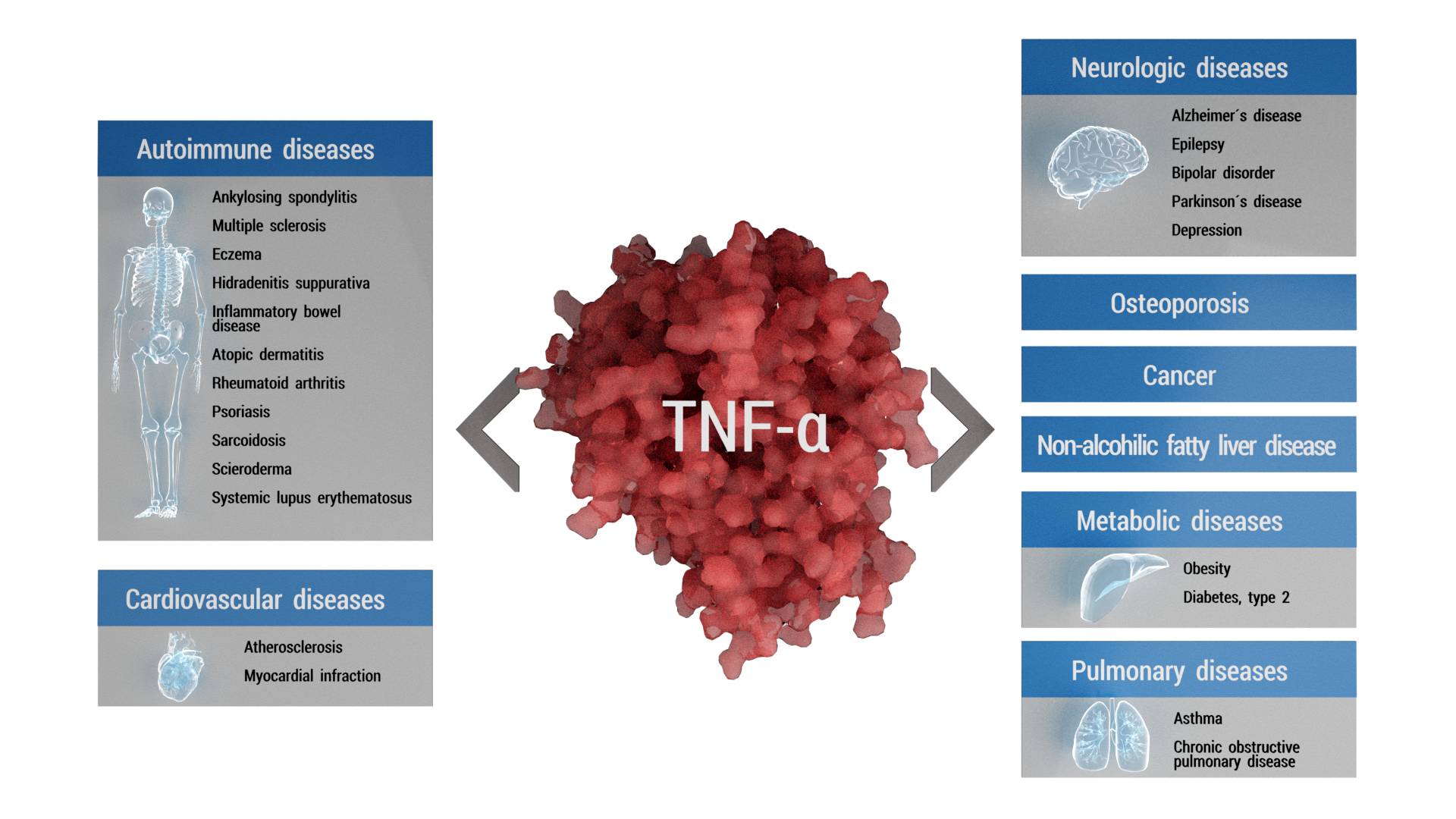 Various diseases that have been closely linked to TNF-α and members of its family (adapted from Aggarwal, 2012).
Various diseases that have been closely linked to TNF-α and members of its family (adapted from Aggarwal, 2012).
REFERENCES
1. Wajant, H., Pfizenmaier, K., Scheurich, P. Tumor necrosis factor signaling, IN: Cell Death Differ., 2003,10(1), p. 45-65.
2. Chu, W.M. Tumor necrosis factor, IN: Cancer Lett., 2013, 328(2), p.222-5.
3. Aggarwal, B.B., Gupta, S.C., Kim, J.H. Historical perspectives on tumor necrosis factor and its superfamily: 25 years later, a golden journey, IN: Blood, 2012,119(3), p. 651-65
4. Gottlieb, A.B. Tumor necrosis factor blockade: mechanism of action, IN: J Investig Dermatol Symp Proc., 2007, 12(1), p.1-4.
"We had a sound experience of working with the Moving Medical Media team. They help make "science fun"!"
Lucia Messingerova, MSc., PhD., research fellow, Centre of Biosciences/ Slovak Academy of Sciences
"Moving Medical Media helped to creatively demonstrate abstract concepts in visually exciting ways..."
Zdena Sulova, Ing., DrSc., director, Centre of Biosciences/ Slovak Academy of Sciences
About Moving Medical Media
- Customized Stereoscopic 3D Animation
- 3D Camera Shooting
- Virtual Reality
- Customized 3D Model
- Hardware solutions